Medical Marijuana Program
By Ronni Burkhart , Assistant Executive Director | 4 years ago
Health & Human Services Analysts: Aniam Iqbal - Budget Analyst , Mara Perez - Senior Budget Analyst
Summary
The Medical Marijuana Program was authorized in 2016 through the enactment of Senate Bill 3, which was codified as Act 16 of 2016 to provide safe access to marijuana for medical purposes. Act 16 designates the Department of Health (DOH) as the lead administrative agency with regulatory authority and jurisdictional oversight over the Medical Marijuana Program. The Commonwealth’s program gives DOH the authority to:
- issue permits to medical marijuana organizations, which include:
- grower/processors authorized to grow and process marijuana into acceptable forms of medical marijuana
- dispensaries authorized to offer for sale and sell medical marijuana to patients or their caregivers
- register physicians to certify serious medical conditions of patients; and,
- issue identification cards to eligible patients or their caregivers.
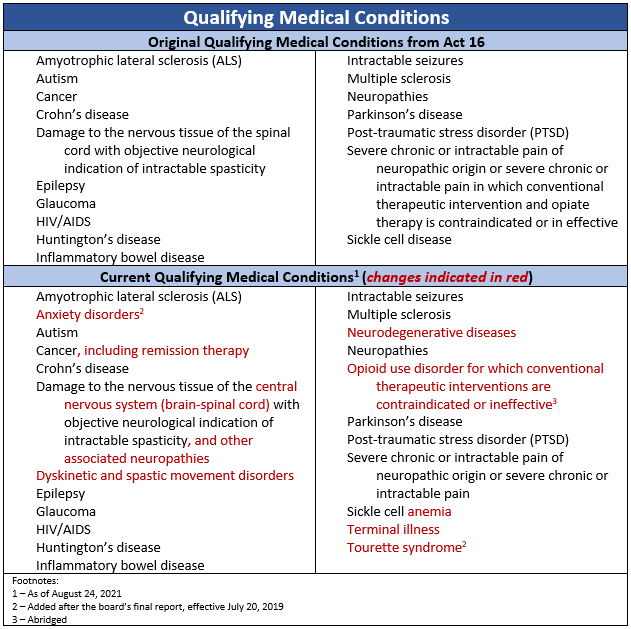
Act 16 created a Medical Marijuana Advisory Board and gives that board the authority, with the approval of the Secretary of DOH, to add diseases to the 17 serious medical conditions initially included in Act 16. The Medical Marijuana Advisory Board also has the authority to recommend and, with the approval of the Secretary of DOH, add additional forms of medical marijuana that can be made available to patients or their caregivers. Finally, the Chapter 20 research provisions are noteworthy in that it allows research to be conducted by academic clinical research centers. The research component enhances the statutory authorization of medical marijuana by ensuring ongoing research in the effective use of medical marijuana to treat serious medical conditions.
This primer describes the creation and evolution of the Medical Marijuana Program and the Medical Marijuana Program Fund.
Establishment
Legislation to create a medical marijuana program in Pennsylvania was first introduced in November 2013. The legislation was inspired by Charlotte Figi, who was born with Dravet Syndrome, which caused her to experience hundreds of seizures per week, and children like her who could benefit from components of cannabis, otherwise known as marijuana. At the time, 21 other states and Washington D.C. had similar laws in effect.
Cannabis is comprised of cannabidiol (CBD) and tetrahydrocannabinol (THC). When cannabis is grown in specific proportions of CBD and THC, users with numerous medical conditions can reap the benefits of the CBD in cannabis with low enough THC levels to bypass the psychoactive effects.
Act 16 passed on April 17, 2016, establishing a medical marijuana program in Pennsylvania. The legislation was all encompassing -- from issuing permits to grow, process, and dispense medical marijuana to defining the serious medical conditions qualifying patients to access medical marijuana. There is no cap on the amount of THC that medical marijuana may contain in Pennsylvania. The statute designated the Pennsylvania DOH as the agency responsible for administering the program.
Medical Marijuana Advisory Board
Chapter 12 of Act 16 established a 15-member board to review and receive feedback on the program in Pennsylvania and review other state programs nationally. As part of its duties, the board was required to issue a report to the governor and General Assembly two years following the effective date of the legislation that addressed the following areas:
- Types of medical professionals that can issue patient certifications
- Qualifying medical conditions
- Form and manner of consumption
- Affordability
The report was issued April 9, 2018. Act 16 permitted the DOH secretary to effectuate recommendations made by the board through publication in the Pennsylvania Bulletin. That notice was published May 12, 2018, agreeing with all 21 recommendations in the report from the board that received a passing vote by members.
One board recommendation in the final report approved by the secretary was the expansion of allowable forms of medical marijuana to include dry leaf or plant form for administration by vaporization. This change was viewed as a way to expand access to medical marijuana and assist with affordability, as this form is the least costly version. Several other major recommendations effectuated are mentioned in context in the sections that follow.
Act 44 of 2021 established the process of board recommendations and effectuation by the secretary as an ongoing process.
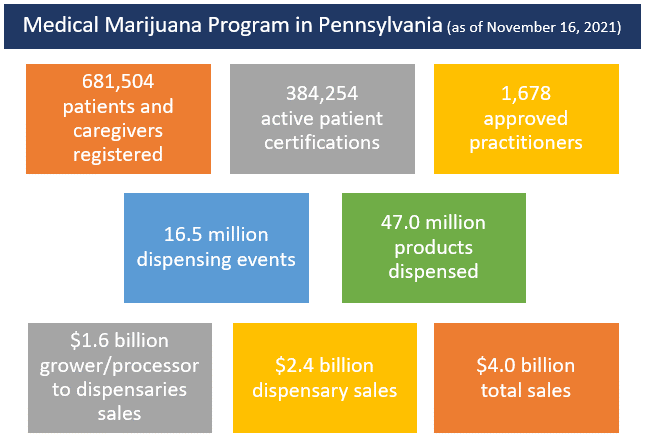
Patients and Caregivers
Pennsylvania residents who have a qualifying serious medical condition must have the condition certified by an approved physician. Patients must also register and pay for an identification card to use that card to access medical marijuana at Pennsylvania dispensaries.
Act 16 included 17 serious medical conditions qualifying Pennsylvanians to access medical marijuana. Through the secretary of DOH effectuating the recommendations of the board’s two-year report, several conditions were updated or added. Additionally, the board recommended they be given authority to continuously review conditions for potential additions and changes. This recommendation was also effectuated by the secretary of DOH. Since then, the board has recommended, and the secretary of DOH has approved, two additional qualifying conditions.
Patient identification cards cost $50; however, individuals who are enrolled in Medical Assistance, PACE/PACENET, CHIP, SNAP and WIC may be eligible for a discount. Identification cards are valid for the duration of the physician’s certification up to one year.
Patients may designate a caregiver to pick up medical marijuana at a dispensary on their behalf. Caregivers are required for patients under 18 and can be especially beneficial for home bound individuals or individuals with transportation barriers. Patients may designate up to two caregivers and originally each caregiver could serve up to five individuals, although Act 44 removed the limit on the number of individuals caregivers could serve. Caregivers must have a valid Pennsylvania issued driver’s license or identification card, be at least 21 years of age (unless approved by DOH), register with DOH, and pass a background check that includes no drug related offenses within the last 5 years.
Physicians
Physicians may register with DOH to become a practitioner eligible to certify a patient as having a qualifying serious medical condition. Physicians must hold a valid Pennsylvania license to practice medicine that may not be expired, revoked, nor suspended. Physicians must also complete a 4-hour training provided by a DOH approved training entity. Additional restrictions were recommended by the Board when certifying a qualifying serious medical condition of a patient under 18 years of age and will be effectuated by the Secretary on a delayed basis when DOH determines a sufficient number of providers exist as to not create an access barrier.
Medical Marijuana Organizations
Grower/Processors
Grower/processors are entities certified by DOH to grow and process medical marijuana. Act 16 limited the number of grower/processor permits to 25 entities and no person was permitted to hold more than one permit. In addition, no more than 5 grower/processors may also be permitted as dispensaries and at no point can more than 20 percent of all grower processors be permitted as dispensaries.
Permittees and their employees must complete a 2-hour training course developed by DOH that includes recognizing unauthorized activity, reporting unauthorized activity, diversion of medical marijuana for unlawful purposes, falsification of identification cards, proper handling, and recordkeeping, as required by Act 16.
Grower/processors may only provide medical marijuana to a dispensary holding a valid Pennsylvania permit. In addition, grower/processors must meet local zoning requirements.
To date, DOH awarded grower/processor permits in two phases in June 2017 and July 2018. There are currently 22 grower/processors operational.
Dispensaries
Dispensaries are entities certified by DOH to dispense medical marijuana to qualified patients and caregivers. Act 16 limited the number of dispensary permits to 50, with each permit allowed to have up to three separate locations. No person was permitted to hold more than five permits.
Permittees and their employees must complete a 2-hour training course developed by DOH with the same topics required of grower/processors. Dispensaries must also always have a physician or pharmacist available in person or virtually in real time while dispensing medical marijuana products but may have a nurse practitioner or physician assistant at satellite locations.
Dispensaries may only obtain medical marijuana from a Pennsylvania permitted grower/processor. In addition to meeting local zoning requirements, dispensaries must also meet the facility requirements in Act 16 including:
- May only dispense medical marijuana indoors or in accordance with DOH established curbside protocols
- Maintain continuous video surveillance and retain recordings for at least 180 days
- May not operate on the same site as a grower/processor
- May not be located within 1,000 feet of a school or childcare center, unless waived by DOH
To date, DOH awarded dispensary permits in two phases in June 2017 and December 2018. There are currently 80 dispensary sites operational.
Laboratories
As a program control, sampling and testing of medical marijuana is performed by DOH-approved laboratories. Samples are collected after harvesting and again after processing. Testing is conducted for contaminants and potency adherence to labeling. There are currently six approved laboratories that conduct this work.
Academic Clinical Research Centers and Clinical Registrants
Act 16 called for, and Act 43 enhanced, a first-in-the-nation medical marijuana research program. The research program matches academic clinical research centers (ACRC) with dual permitted grower/processor and dispensary medical marijuana organizations to enhance efforts to determine how medical marijuana can be best used to effectively treat serious medical conditions.
Eight universities were certified by DOH as ACRCs in September 2018 and one ACRC was added in September 2021. Those entities are:
- Drexel University College of Medicine – Philadelphia
- Lewis Katz School of Medicine at Temple University – Philadelphia
- Penn State College of Medicine – Hershey
- Sidney Kimmel Medical College at Thomas Jefferson University – Philadelphia
- Perelman School of Medicine at the University of Pennsylvania – Philadelphia
- University of Pittsburgh School of Medicine – Pittsburgh
- Lake Erie College of Osteopathic Medicine – Erie
- Philadelphia College of Osteopathic Medicine – Philadelphia
- Geisinger Commonwealth School of Medicine – Scranton (2021)
To date, DOH has approved clinical registrants in two rounds in June 2019 and in February 2020. There are currently seven clinical registrants. Once approved as a clinical registrant, the permitting is changed freeing the grower/processor and dispensary permits for other applicants.
One original ACRC, University of Pittsburgh School of Medicine, remains available to be partnered with a clinical registrant. Act 44 of 2021 expanded the number of permissible clinical registrants from eight to ten.
Act 44 also established a procedure for ACRC research proposal submission and approval by DOH, including timelines for proposal submission, approval, and completion of research.
Regulations
Act 16 provided DOH 18 months to promulgate temporary regulations that were permitted to be in effect for two years. Temporary regulations were adopted effective October 29, 2016, and were to expire October 29, 2018. The bulk of the regulations were then amended effective May 17, 2018, pushing the expiration date to May 12, 2020. Then, due to the COVID-19 pandemic, Act 10 of 2020 pushed the end date of temporary regulations to November 20, 2021, and Act 44 of 2021 further extended the expiration date of the temporary regulations to May 31, 2022.
DOH submitted proposed regulations to the Independent Regulatory Review Commission (IRRC) on February 16, 2021, and established a public comment period from March 6, 2021, to April 5, 2021. Final regulations are due to IRRC no later than April 5, 2023, according to the regulatory process, although the statutory requirement highlighted previously dictates they must be in place sooner.
Funding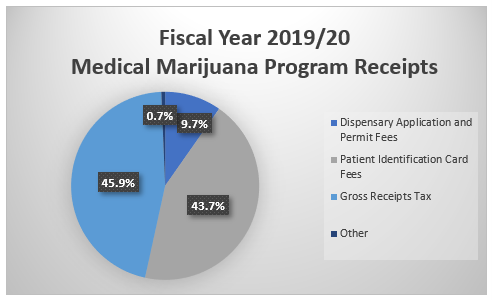
Act 16 also established the Medical Marijuana Program Fund to collect application fees, permit fees, patient identification card fees, and a gross receipts tax on medical marijuana sold by grower/processors to dispensaries.
Act 16 established that each grower/processor was to pay a non-refundable application fee of $10,000, initial permit fees of $200,000, and an annual renewal fee of $10,000 covering all locations.
The Act also established that dispensaries were to pay a non-refundable application fee of $5,000, initial permit fee of $30,000 per location, and an annual renewal fee of $5,000 covering all locations.
Initial permit fees were refundable for non-selected applications and annual renewal fees are refundable if a renewal is not granted by DOH.
Act 16 also established a five percent gross receipts tax on all medical marijuana sales from grower/processors to dispensaries.
DOH is permitted to monitor the market price of medical marijuana and may work with the Department of Revenue to implement price caps for up to 6 months, with unlimited renewals, if prices become excessive.
The Fund received a loan from the General Fund of $3 million dollars to begin operations. Upon repayment of that loan, the distributions outlined in Act 16 are to be implemented. They are:
- 40 percent for program operations (DOH),
- 15 percent for patient affordability assistance programs (DOH),
- 30 percent for medical marijuana research programs (DOH),
- 10 percent for drug misuse prevention, counseling, and treatment (Department of Drug and Alcohol Programs), and
- 5 percent for distribution to local police departments (Pennsylvania Commission on Crime and Delinquency).
The loan will be repaid in the 2021/22 fiscal year triggering the above allocations.
National Landscape
Nationally, as of May 2021, 36 states and 4 territories allow for the medical use of cannabis.
At the national level, marijuana remains a Schedule I substance under the Controlled Substances Act, meaning federally it remains classified as having no accepted medical use. Therefore, even though medical marijuana is legal in Pennsylvania, national laws still create barriers, including financial limitations for the permitted business and travel restrictions for patients going across states lines.
Relevant Legislation
Established the Medical Marijuana Program
Expanded the research program within the Medical Marijuana Act
Extended the temporary regulation expiration date to November 20, 2021
Act 44 of 2021 – Medical Marijuana Act Amendment
Numerous updates to the original enacting legislation based on the DOH two-year report and extended the temporary regulation expiration date to May 31, 2022